The RAMER Reviews: Prehospital Tranexamic Acid for Severe Trauma - The PATCH-Trauma Trial
Written by: Boyd Qu, MD; Edited by: Timothy Khowong, MD, MSEd
TL;DR: Tranexamic acid given in the prehospital setting for patients at risk of trauma-induced coagulopathy did not have significantly improved functional outcomes or mortality at the 6-month mark. There may be a mortality benefit at the 24 hour and 28 day mark. There was no significant difference in thromboembolic events in the TXA group.
Background:
TXA is a hot topic in medicine right now and a drug that’s been heavily researched in all kinds of possible bleeding scenarios. Most of the studies have been negative in terms of improving outcomes so far, except for a positive signal in the CRASH-2 trial.
This PATCH-Trauma trial is probably the closest study we’ve had thus far that could possibly replicate the CRASH-2 trial results so let’s dive into it.
The Study:
This was a double-blind, randomized, placebo-controlled trial that attempted to answer the following question: does prehospital administration of tranexamic acid (TXA) increase the likelihood of survival with a favorable functional outcome among patients with major trauma and suspected trauma-induced coagulopathy?
Inclusion criteria included adults >18 years of age with suspected severe traumatic injuries with a COAST score of >3. Patients also needed to be treated at the scene by paramedics or physicians and transported by road/air ambulance. Exclusion criteria included any known or suspected pregnant patients and nursing home/assisted living patients.
The study wanted to hone in on patients who were at risk of trauma-induced coagulopathy (sickish trauma patients) and they did this by calculating a Coagulopathy of Severe Trauma (COAST) score which you can see below.
Patients were randomly assigned in a 1:1 ratio to receive either TXA or placebo. The TXA group received a 1g bolus over 10 minutes as soon as possible en route to the hospital and another gram infused over 8 hours at the hospital.
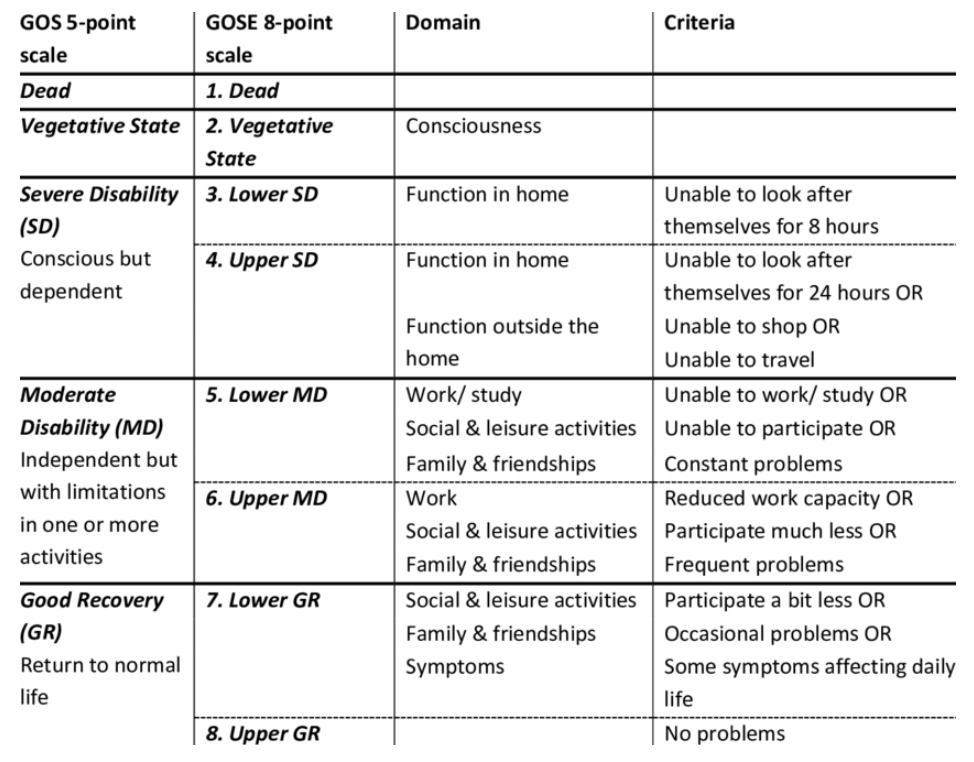
Primary outcome was survival with favorable functional outcome at 6 months. They used the Glasgow Outcome Scale-Extended questionnaire administered by telephone interviewers. A score of >5 was considered a “good” outcome.
Secondary outcomes included death at 24 hours, 28 days, and 6 months after injury as well as the rates of vascular occlusive events (DVT, PE, MI, Stroke). Patients were analyzed in an intention-to-treat analysis.
The study found no significant differences in favorable functional outcomes at 6 months between either group. A GOS-E level > 5 was recorded in 307/572 (53.7%) patients in the TXA group vs. 299/559 (53.5%) in the placebo group (RR 1.00; 0.90 - 1.12). There was a significant reduction in the number of deaths at 24 hours after injury (RR 0.69; 0.51 - 0.94) and 28 days after injury (RR 0.79; 0.63 - 0.99).
There were no significant differences in vascular occlusive events. The results are summarized below with the significant results outlined in red.
A couple of caveats to keep in mind: 13% of the patients were lost to followup. There were a lot of protocol violations with 15% of the placebo group actually receiving TXA and 21% of the TXA group who didn’t end up receiving the infusion dose. The paper also doesn’t go over how many people were screened. If 10,000 people were screened and only 1,300 people were included in the study that would be very concerning. There also wasn’t nearly enough penetrating trauma (around 7% total in both groups).
Conclusion:
My personal takeaway from this paper is that TXA at the very least is not causing more harm. There’s only been one positive study thus far in the trauma world (CRASH-2) and this paper comes the closest to trying to replicate the results. The results aren’t very impressive and TXA isn’t going to be the miracle drug we want it to be, but it could matter in those edge cases. I’m going to keep recommending that we give TXA for those sick trauma patients based on what we know so far.